|
Notice: Since February of 2019, this website has seen very erratic drops in search engine referrals. So if you consider the information on this page to be worthwhile, I recommend posting a link to the page where you can. Otherwise it is unlikely that it is going to reach very many people. For more on that, see About Dave Conklin.net. 2/23/20, last rev 10/14/21. The Goal I contemplated putting page like this together several years ago (2009). I made a rough sketch and promptly threw it on the shelf, to post if I ever got the time. I knew there was a lot of people and institutions working on such technology, but by the sound of things, “the goal” might be achievable much sooner then I ever expected. The goal is energy independence for the typical homeowner, and not just energy independency, but energy from a source that is safe, affordable, and emits zero CO2. Power companies constantly preach conservation, then, of course, they turn around and raise their rates through the roof so you never save any money, hack up your nice carbon-capturing trees to protect their lines, and produce CO2, coal ash and nuclear waste like they are going out of style (if coal or nuclear plants are involved). And half of the power they produce is wasted in transmission. And then, of course, there is the whole smart-meter issue. So the only solution, as I see it, to make them go out of style. The idea here is simple: Below the illustration is a brief summary of where the progress stands for each category of technology mentioned in the illustration. As mentioned, there many people working furiously to improve these technologies. I’m sure it is not going to be easy keeping things up to date. |
|
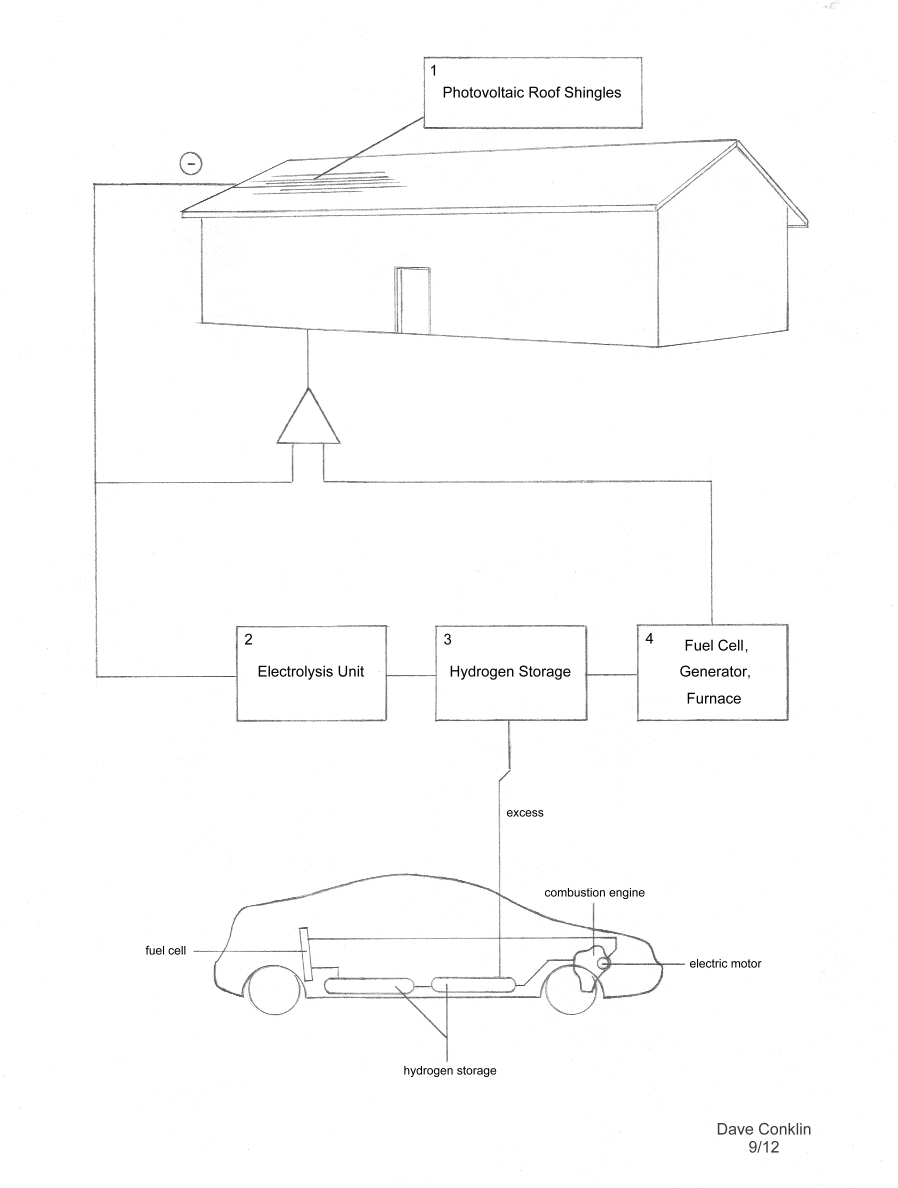
First off, I just want clarify something. In researching some of this, I came across a few Internet articles that state how current flows from the cathode of a battery to the anode. What is often the case, however, is the author fails to mention that current flow is a myth and that electrons flow from the anode (the negative terminal) of a battery to the cathode. And for any electronic device, the terminal receiving electrons is the cathode. Why the industry does not correct this potentially confusing blunder that only has to do with wiring schematics, and has nothing to do with reality, I will never know. 1 Photovoltaic Roof Shingles: The idea of a roof covering that doubles as an energy collector just makes a lot of sense to me. The problem with solar panels is, they’re delicate, they’re expensive, and a couple expensive panels do not utilize the entire roof area. What I’m talking about is something tuff, flexible, and relatively inexpensive; something that can stand up to a moderate hail storm (like any good shingle) and not cost a fortune to replace if it does happen to get damaged. Solar shingles have been around a while, but had the reputation of being more delicate, less efficient, and even more expensive to install than a panel. In 2009, Dow Corporation came out with shingle that better fit some of these criteria at the sacrifice of efficiency. Still no leap in efficiency, but a project at Cal Tech headed by one Mr. Nate Lewis has recently come up with a flexible collector that promises to be cheaper than the Dow shingles by using leaf-like veins of impure silicon, as opposed to the solid sheets of pure silicon normally making up photocells. At present, the efficiency needs to go up, the price—down. 2 Electrolysis Unit: Of course, once you get the power flowing, you have to store it somewhere. The status quo has been the lead-acid battery, which, of course, contains gobs of heavy metals and has an expiration date. An alternative is converting the extra power to hydrogen, which—of course—has no expiration date, and storing it. The conversion is done by running the electric current through water, and separating the hydrogen from the oxygen—not a particular easy thing to do. You can use pretty much any old type of electrode to produce hydrogen, but to boost the efficiency of the process to anything practical, a catalyst must be applied to the electrodes. The status quo has been to use expensive platinum, and that has been a problem. Again, it’s Cal Tech to the rescue—well, maybe; their new collector can also be used for electrolysis, but from what I gather, it lacks efficiency. On the plus side, the entire process of sunlight to hydrogen can be made more efficient (10% is the latest figure) by allowing the sunlight-to-electrolysis process to take place all at once (letting the light shine on a catalyst coated photocell while it is in water) but doesn’t seem too practical for roof shingles. Still, it might be a technology to pay close attention to. According to the Wikipedia article on the electrolysis of water, the efficiency of electrolysis can range from 50-80%. So if a power plant were to use all of its power for electrolysis instead of transmitting it, there would probably be no significant loss of energy by doing so, even when the transport of the hydrogen is added to the equation. And as domestic power production becomes more and more widespread, that would mean greater efficiency overall in energy production. 3 Hydrogen Storage: If a safe and more efficient method of hydrogen storage were discovered, it would truly open a world of possibilities. After coal and nuclear power stations are phased out and the transmission lines are gone, electricity from other sources—hydroelectric power, solar arrays, wind, and space power etc.—could be used for electrolysis. The hydrogen could then, of course, be used to power our transportation and supplement any domestic energy needs not met by solar power etc. Traditionally, the problem with storing hydrogen in any usable amount is that very high pressures are involved. A vehicle, for example, using conventional hydrogen storage would need a large and tremendously heavy tank to have the range of a gasoline powered vehicle. Hydrogen can be stored in the form of ammonia. Ammonia itself does not burn, so the hydrogen must again be separated out. The fact that ammonia does not burn and would not require a heavy pressurized tank makes it attractive as an automotive fuel, but both processes—producing ammonia and then liberating the hydrogen—require energy and or a catalyst. And raw ammonia is very nasty stuff if a tank were to rupture. The idea has been around for a while to store the ammonia using certain materials that soak up the ammonia like a sponge. But the idea is also being applied to hydrogen itself. The pinnacle of this technology, in my opinion, has to be the idea of using burnt chicken feathers for the job. Gosh! where are we going to find chicken feathers? All levity aside, the beauty in the idea has to be in the commonplace of bird feathers and therefore the inexpensiveness of the technology. At any rate, storing hydrogen is, at present, probably more practical for domestic use than for autos. 4 Fuel Cell: Extracting the energy from hydrogen can be as simple as striking a match to it: A specialized furnace can be constructed to burn hydrogen for domestic heating. Burning it in a combustion engine is about 60% efficient. And, of course, combustion engines can be used to propel a vehicle or turn an electric generator. Such a generator could be ran inside a house, as the only byproduct is water. Another way is to use the hydrogen in a fuel cell to produce electricity. The type of fuel cell we are talking about here is a PEM (Proton Exchange Membrane) fuel cell. Fuel cells are sort of like an electrolysis cell in reverse in regards to the fact that you put fuel in and get electricity out. But again, a big hurdle has been the expensive platinum catalyst needed on the electrodes. The efficiency of a typical hydrogen fuel cell is not great, around 50%, but here is something to always keep in mind: Although some energy is lost in both processes—electrolysis cells and hydrogen fuel cells—most of that lost energy is in the form of heat. Same goes for burning hydrogen in a combustion engine for that matter. If a person living in a cold climate used that heat to directly heat their house, it would be an easy way to make use of that heat, and the efficiency of the entire system goes way up. At any rate, the search goes on for a cheaper alternative electrode/catalyst material with equal or greater efficiency to platinum. Every so often, there is a newsflash regarding a breakthrough in potential-catalyst materials. These usually center around metal alloys containing cobalt. For example: A group at MIT announced in 2008 the discovery of an efficient material of carbon, iron and cobalt. But this was only for the cathode of a PEM; the anode—where the hydrogen is ionized—is a bit more challenging, but less is always better. Commercial hydrogen: The challenge, of course, is to produce hydrogen commercially without producing CO2, coal ash or nuclear waste. You might be surprised to learn that there is another method in addition to using straight current from solar arrays etc. to extract hydrogen from water. Another more efficient al-be-it slower method of producing hydrogen is the microbial electrolysis cell (MEC), which uses a tiny amount of current and bacteria to produce hydrogen. The bacteria need some sort of biomass (sugars) or organic acid (vinegar) as a food source, and unfortunately, the end product is not only hydrogen, but CO2. There are other bacteria that produce hydrogen, along with the organic acids needed by the first bacteria, from biomass; again, with CO2 as a biproduct. Every so often there is a news flash regarding some new breakthrough in the field that centers on a new bacteria, photo-bacteria, or even simple enzymes to liberate hydrogen, but what to do with all the CO2? A complex system has been worked out that serves to lock up the CO2 in oily hydrocarbon-containing lipids produced by algae, which use CO2 for food. Of course, the oil would then have to be permanently stowed away somewhere to truly get rid of the CO2. A few related links: |
HOME
mysteries, commentary, sci-fi